Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Tateno, Haruka; Sato, Takumi; Tsubata, Yasuhiro; Hayashi, Hirokazu
no journal, ,
Japan Atomic Energy Agency has been pursuing research and development on partitioning and transmutation of minor actinides (MAs) by accelerator-driven system (ADS) using uranium-free nitride fuel. The target transmutation ratio of 99% MAs can be achieved if 99.9% of MAs in the spent fuel are recovered in reprocessing. From the point of view of neutronics design, the weight ratio of rare-earths against MAs in the refabricated fuel should be 5.0% or less. In this study, we calculated the material balance in the pyro-reprocessing of nitride fuel to evaluate the processing conditions to meet the above-mentioned requirements.
Tsubata, Yasuhiro; Sugawara, Takanori; Hayashi, Hirokazu
no journal, ,
Compositions of minor actinide (MA) nitride fuel irradiated in accelerator driven system was estimated. In burnup calculation ADS3D program developed by JAEA was used. The compositions of the MA nitride fuel of the 1st cycle after irradiation (thermal power: 800 MW, irradiation period: 600 days, cooling time: 4 years) was compared with those before irradiation. Reduction rate of the minor actinides (MA; Np, Am, Cm) was 29 at.%, and that of actinides (MA, U, Pu) was 16 at.% at the central part of the inner region. Reduction rates were smaller at the bottom of the outer region (12 at.% for MA, 5 at.% for An). The compositions of fission products were 5.4 at.% at the central part of the inner region, 3.6 at.% at the bottom of the outer region. Estimation of the chemical forms of FP elements based on the calculation of the spent fuel composition is in progress.
Hayashi, Hirokazu; Sato, Takumi
no journal, ,
Transmutation of long-lived radioactive nuclides including minor actinides (MA: Np, Am, Cm) is effective to reduce the burden of high level radioactive wastes and using repositories efficiently. Uranium-free nitride fuel has been chosen as the first candidate fuel for MA transmutation using accelerator-driven system (ADS) in Japan Atomic Energy Agency (JAEA) under the double strata fuel cycle concept. To improve the transmutation ratio of MA, reprocessing of spent MA fuel and reusing the recovered MA is necessary. Our target is to transmute 99% of MA arisen from commercial power reactor fuel cycle, with which the period until the radiotoxicity drops below that of natural uranium can be shorten from about 5000 years to about 300 years. Each reprocessing process is required to recover 99.9% of MA to meet the target. Typical composition of the solid solution type (MA,Pu,Zr)N fuel is considered as 30 wt.% of MA nitride, 20 wt.% of Pu nitride, and 50 wt.% of ZrN (dilution material to adjust the power density). Pyroprocessing has been proposed to adopt for reprocessing of the spent MA nitride fuel. This paper summarizes the status of our study on pyroprocessing of ZrN-based nitride fuels.

Akashi, Shin; Shibata, Hiroki; Sato, Takumi; Hayashi, Hirokazu
no journal, ,
It is important to evaluate the thermodynamic stability of TRU-Cd intermetallic compounds to select the detailed conditions of the nitridation-distillation combined process. In this study, Gibbs free energy of formation of gadolinium (Gd)-Cd alloy which is used as a surrogate of the TRU-Cd alloy was measured. Gd-Cd alloy was prepared by heating Gd metal with Cd metal (atomic ratio=1:10) in a tungsten crucible sealed in an evacuated quartz tube at 773K for 4 hours. The prepared alloy was identified as the mixture of GdCd and Cd with X-ray diffraction measurement of the filed samples at room temperature. The potential of Gd-Cd alloy sample set in a tungsten crucible versus Ag/AgCl reference electrode was measured in (LiCl-KCl)
and Cd with X-ray diffraction measurement of the filed samples at room temperature. The potential of Gd-Cd alloy sample set in a tungsten crucible versus Ag/AgCl reference electrode was measured in (LiCl-KCl)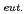 -GdCl
-GdCl at 673-923 K. The electromotive force (
at 673-923 K. The electromotive force (
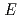 ) of the cell (Gd
) of the cell (Gd  (LiCl-KCl)
(LiCl-KCl)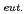 -GdCl
-GdCl
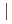 Gd-Cd) was calculated from the difference of the measured potential of Gd-Cd alloy sample and that of Gd metal. The latter was deposited on a tungsten electrode by potentiostatic electrolysis in (LiCl-KCl)
Gd-Cd) was calculated from the difference of the measured potential of Gd-Cd alloy sample and that of Gd metal. The latter was deposited on a tungsten electrode by potentiostatic electrolysis in (LiCl-KCl)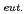 -GdCl
-GdCl . Gibbs free energies of formation (
. Gibbs free energies of formation (
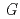

 ) of GdCd
) of GdCd calculated with the equation (
calculated with the equation (
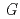

 (GdCd
(GdCd )= -3
)= -3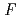

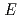 ) agree well with our data obtained by the electrochemical technique using the sample formed by co-deposition of Gd and Cd in a molten salt and the reported data obtained by Cd vapor pressure measurement. This result shows that method of this study is valid for the measurement of
) agree well with our data obtained by the electrochemical technique using the sample formed by co-deposition of Gd and Cd in a molten salt and the reported data obtained by Cd vapor pressure measurement. This result shows that method of this study is valid for the measurement of 
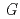

 (GdCd
(GdCd ).
).
Sato, Takumi; Hayashi, Hirokazu
no journal, ,
Nitride formation reactions of 2 wt% Dy-Cd and 2 wt% Gd-Cd alloys in N gas stream have been studied using an apparatus developed for 100 g-Cd scale nitridation tests in order to obtain experimental data required to design industrial scale equipment of pyrochemical reprocessing of MA transmutation nitride fuel. Dy and Gd were used as surrogate materials of MAs and Pu. Most of Dy and Gd dissolved in Cd were converted to DyN and GdN at 1073 K, respectively. By additional heating of the products, Cd concentration deceased to 0.065-0.12 wt%, and recovery yield of Cd was 100%.
gas stream have been studied using an apparatus developed for 100 g-Cd scale nitridation tests in order to obtain experimental data required to design industrial scale equipment of pyrochemical reprocessing of MA transmutation nitride fuel. Dy and Gd were used as surrogate materials of MAs and Pu. Most of Dy and Gd dissolved in Cd were converted to DyN and GdN at 1073 K, respectively. By additional heating of the products, Cd concentration deceased to 0.065-0.12 wt%, and recovery yield of Cd was 100%.